The Brafman Lab is apart of ASU’s School of Biological and Health Systems Engineering located in one of the Interdisciplinary Science & Technology Buildings on campus. In our lab, we primarily use human induced pluripotent stem cells (hiPSCs) to develop model brain cells such as neurons, astrocytes, and microglia. Since these cells cannot be easily acquired and later cultured from patients, differentiating stem cells allows us to study Alzheimer’s related genetics on living brain cells.
Through the use of genetic engineering, such as our personally developed TREE (Transient Reporter in Editing Enrichment) system, we can generate isogenic cell lines that differ in as little as a single nucleotide in their genetic code. This is helpful for our study of the APOE gene, which has three isoforms appearing to have varying effects on a person’s likelihood of developing Alzheimer’s. Between the three isoforms, only two amino acids change; by creating identical cell lines only differing in the APOE gene, we aim study the protective measures of the APOE2 isoform by comparing beta-amyloid accumulation, Tau protein levels, and other disease related phenotypes to the other versions of APOE.
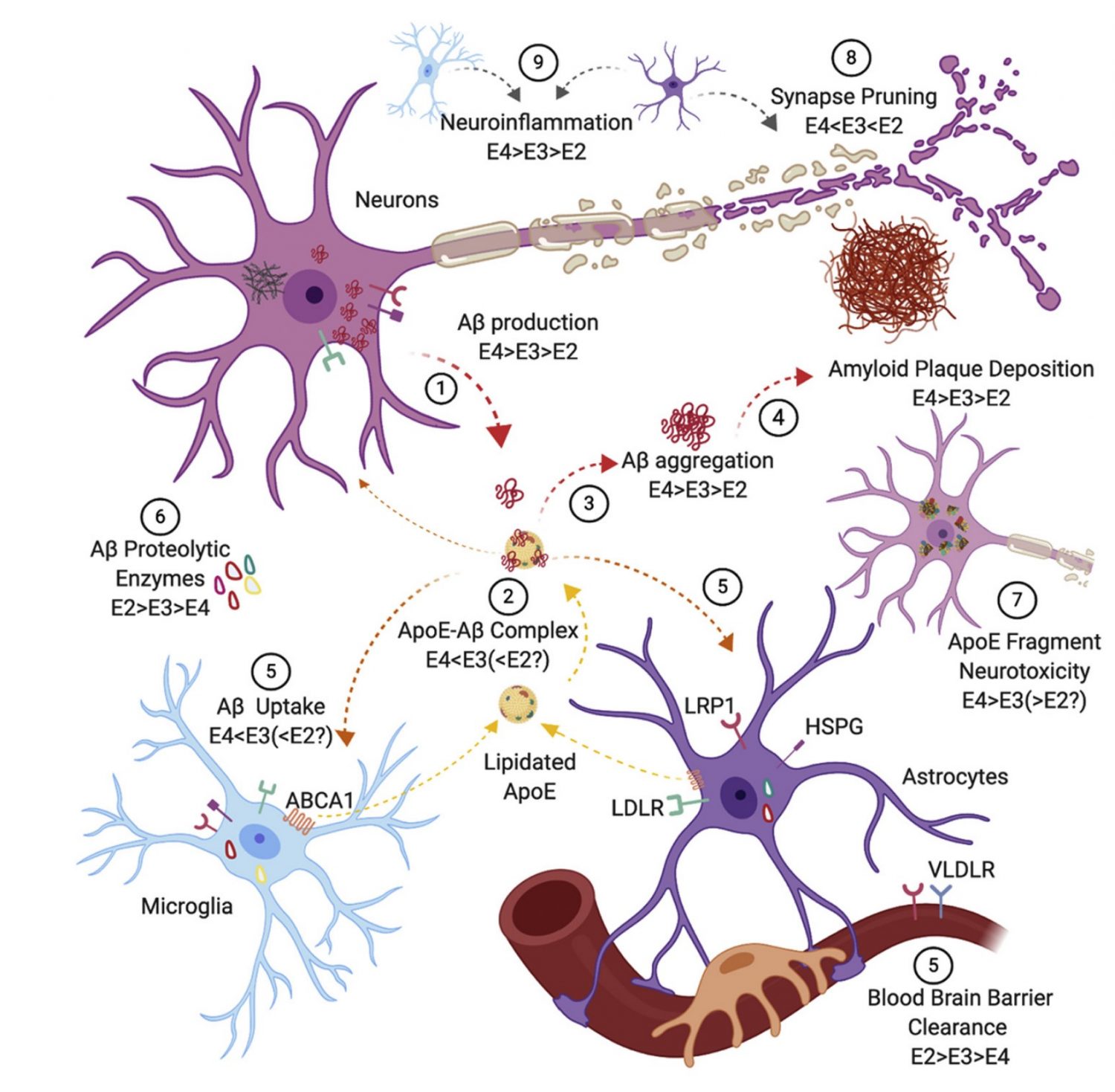
mechanisms by which Apolipoprotein E (APOE) contributes to Alzheimer’s
disease (AD) risk” (2020)
In addition to our Alzheimer’s research, the Brafman lab works on developing and optimizing differentiation protocols for stem cells and studying the mechanisms that determine the pluripotency of cells. The canonical WNT signaling pathway is involved in numerous, complex biological processes, aiding in tissue integrity and immune cell homeostasis; disruption of this pathway is associated with disease and cancer progression. WNT ligands trigger a response to release ß-catenin, a signal transducer, that will interact with DNA-binding proteins in the nucleus and activate the transcription of WNT targeted genes. These targeted genes often are associated with stem cell proliferation and differentiation, and could potentially hold information on how to more effectively study these unique cells in their primal state.
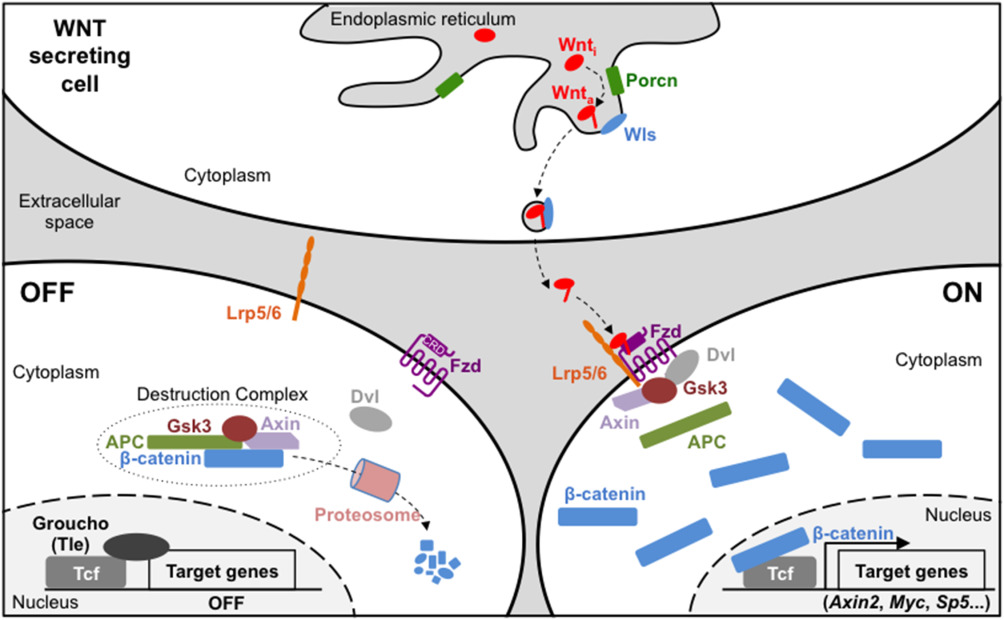
Figure 2 from “Wnt/β-catenin signaling during early vertebrate neural development” (2017)

