Research

Overview
Because of the nature of intermittent, regional limitation, volatility of renewable energies, it is critical to explore diverse electrochemical energy storage devices to fufill various demands of consumers. Specifically, portable devices and transportations require a lighter, safer, higher capacity, chipper, and longer cycling life battery, which put forward a series of challenges to the battery community. Thus, the mismatch between research and commercialization creates opportunities both in academia and industry. Our research is aiming to leverage the practical applications through engineering/developing advanced electrode materials. Meanwhile, it is important to understand fundamental questions in batteries to facilitate devices’ design. The mission of the MU Lab is to ignite the major technological breakthroughs in diverse energy storage devices by understanding electronic/crystal structures, interfacial chemical/physical properties, and electron/ion transport in advanced materials.
1. Manipulating the interfacial chemistry in electrochemistry energy storage devices

The ever-increasing demand for stationary energy storage drives the prosperous investigation of rechargeable batteries. On the way to commercialization, the long-term cycling stability is a significant roadblock. Besides the bulk property maintenance, major challenges in next generation batteries are closely related to interfaces. In a battery, interfaces (between two or three phases) exist everywhere and dominate the whole battery performance.Upon charging, alkali metal ions are extracted from the cathode lattice, driven into the electrolyte, intercalated into, or plated on the anode. Side reactions take place at the interfaces of cathode-electrolyte and anode-electrolyte, form an unstable CEI and SEI layer. Meanwhile, cathodes’ transition metal cations migrate to anode side, disrupting the SEI formation. Understanding the interfacial chemistry in rechargeable batteries is an essential step towards unlocking the underlying fading mechanisms, which is expected to enable rational design of battery materials that ultimately enriches the knowledge and accelerates the commercialization of this technology for broader applications. Thus, how to engineer or rational design the cathode-electrolyte and anode-electrolyte is extremely critical to improving the battery cycle life.
2. Exploring low-cost and high-performance materials
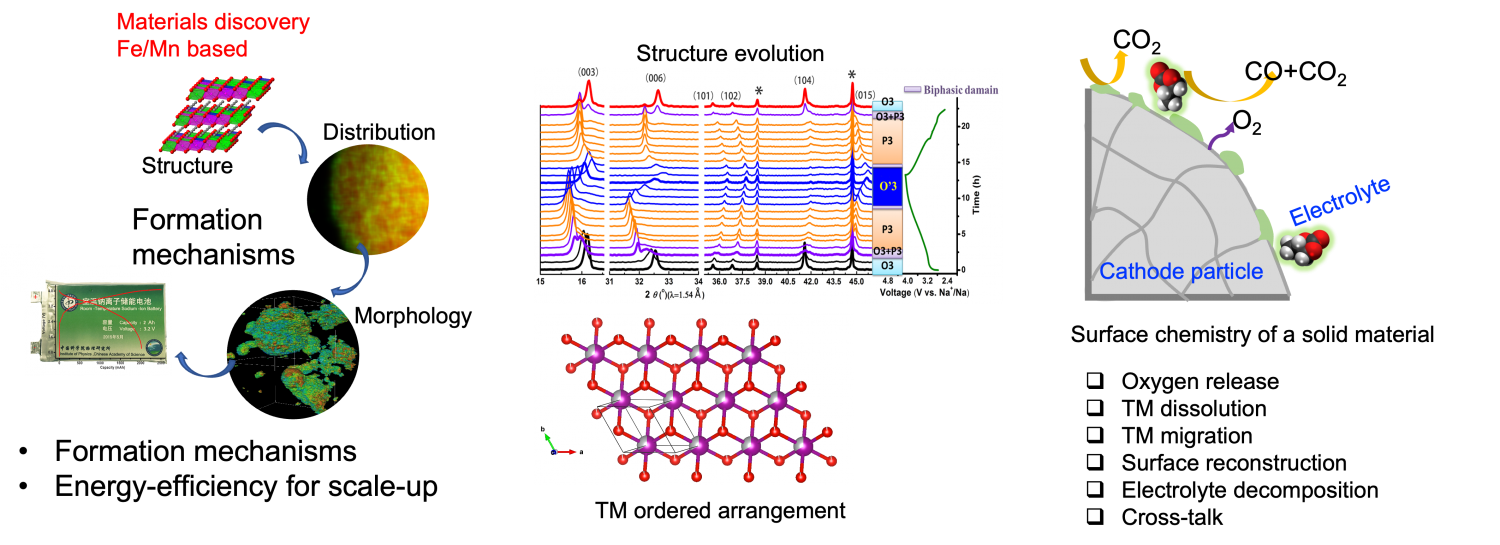
The current commercialized NMC cathode materials encounter the shortage of raw materials reserves and price increasing. Thus, it is critical to explore more possibilities cathode materials with low-cost and earth-abundant elements containing. Introducing earth-abundant elements would relieve anxiety. Exploring novel electrode materials evolves understanding the composition-structural dependency, formation mechanisms, structural evolution, redox chemistry, degradation mechanisms.
3. Developing in situ techniques
The battery system with multiple components complex the understanding of battery failure. For years, significant efforts have been devoted on developing advanced characteraizations for understanding the degradation mechanisms. Amongst these methodes, In situ techniques can monitor the dynamic process of battery materials. However, challenges are still present in many cases. For instance, the mechanical property evolution, one critical determining factors, in solid-state batteries is lack of understanding. There is no reasonable method that can tracking its pathway in individual components. Thus, we will optimize the exiting battery structures to facilitate the application for in situ methods.

Funding Sources

